OUR RANGE OF PRODUCTS
All productsNEWS

Summit Medical Ltd Announces MDR Certification Success, Reinforcing Commitment To High Standards And Patient Safety
Summit Medical Ltd, a global leader in the provision of quality medical products manufactured in the UK, proudly announces today the successful attainment of the European Medical Device Regulation (MDR) certification.
Find out more
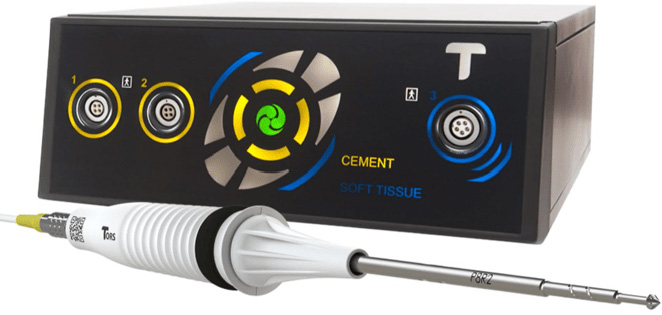
Summit Launch TORS
We are proud to announce our partnership with TORS to bring you the latest in cement removal technology that enhances our Hip Revision portfolio along side EZX and preformed cement spacers.
Find out moreMODERN CEMENTING TECHNIQUE
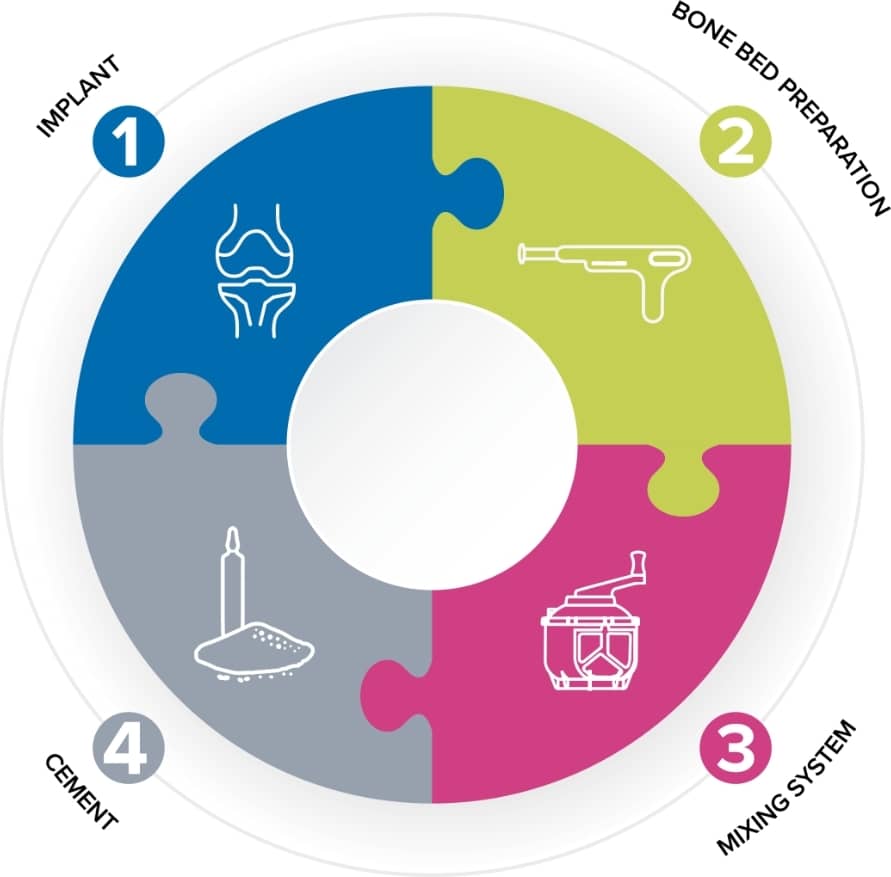
Helping to improve the survivorship of orthopaedic implants
The skill of the surgeon and the quality of the implant are not the only key factors in ensuring a successful joint replacement. The perioperative practitioner is a vital link in the chain.
Good quality bone cement is essential for long-term implant survival, and the role of the theatre nurse in preparing that cement is vitally important. Cement mantle failure is the primary cause of aseptic loosening which is the most common indication for hip revision1. With the population getting older and staying active for longer, the survival rate of joint replacements is becoming even more important.
Ref 1: Nicholas A. Bedard MD, John J. Callaghan MD, Michael D. Stefl MD, Steve S. Liu MD Published online: 20 August 2014. The Association of Bone and Joint Surgeons 2014 Systematic Review of Literature of Cemented Femoral Components: What Is the Durability at Minimum 20 Years Followup?
GLOBAL TRUSTED PARTNERS
We are proud to partner with following global leading orthopaedic companies to design, manufacture and supply large joint, small joint and biologics products.